News
Ingestible devices are often used to study and treat hard-to-reach tissues in the body. Swallowed in pill form, these capsules can pass through the digestive tract, snapping photos or delivering drugs.
While in their simplest form, these devices are passively transported through the gut, there are a wide range of applications where you may want a device to attach to the tissue or other flexible materials. A rich history of biologically inspired solutions exist to address this need, ranging from cocklebur-inspired Velcro to slug-inspired medical adhesives, but the creation of on-demand and reversible attachment mechanisms that can be incorporated into millimeter-scale devices for biomedical sensing and diagnostics remains a challenge.
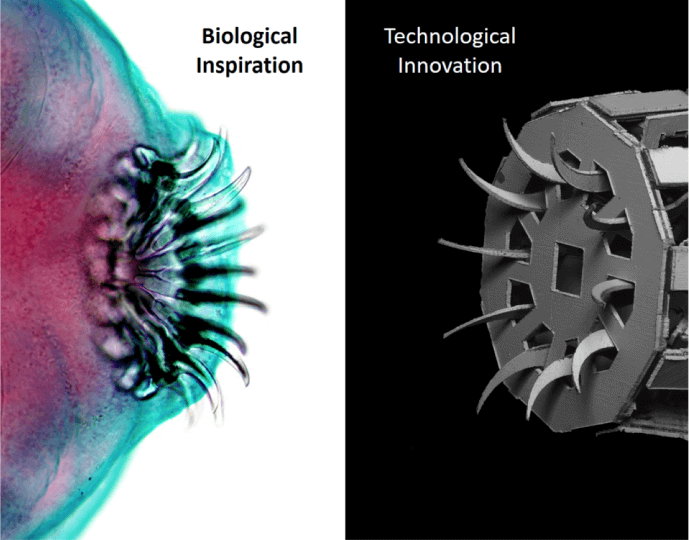
A new interdisciplinary effort led by Robert Wood, the Harry Lewis and Marlyn McGrath Professor of Engineering and Applied Sciences in the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and James Weaver, of Harvard’s Wyss Institute, has drawn inspiration from an unexpected source: the world of parasites.
"Parasitic species have a rather dubious reputation with the general public due to their often terrifying body forms and unfamiliar lifecycles that seem straight out of science fiction movies,” said Weaver. “Despite this fact, it is important to realize that these species are particularly well adapted for anchoring into a wide range of different host tissue types using a remarkably diverse set of species- and tissue-specific attachment organs. These features make them ideal model systems for the development of application-specific synthetic tissue anchoring mechanisms for biomedical applications.”
“Mimicking both the morphology and functionality of these complex biological structures is an incredibly challenging problem, and requires expertise from a wide range of fields including robotics, microfabrication, medical device design, and invertebrate zoology,” said Wood.
The research is published in PNAS Nexus.
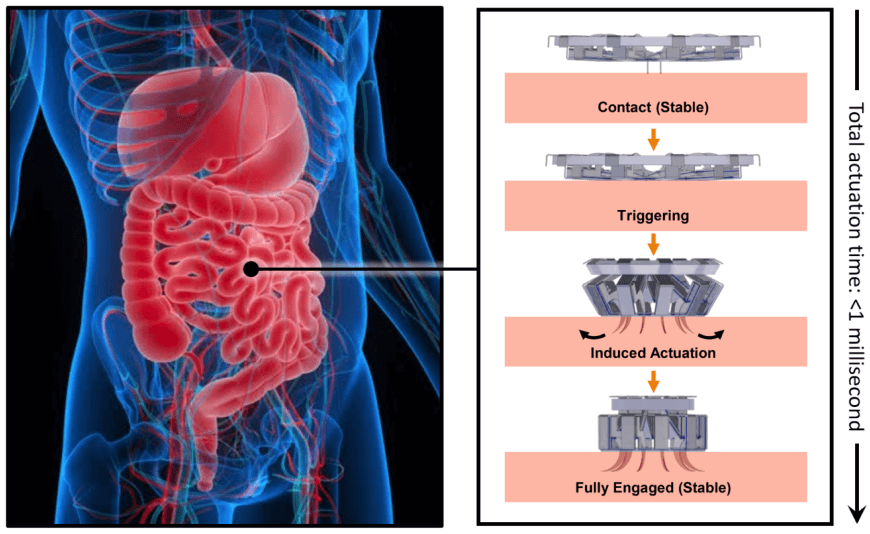
Flow chart illustrating the mechanism of action of the tapeworm-inspired tissue anchoring mechanism. Upon contact with a tissue surface (in this case, the intestinal lining), the small protruding trigger posts (top right image) are depressed, rapidly deploying the curved array of hooks which penetrate the tissue surface (right bottom three images).
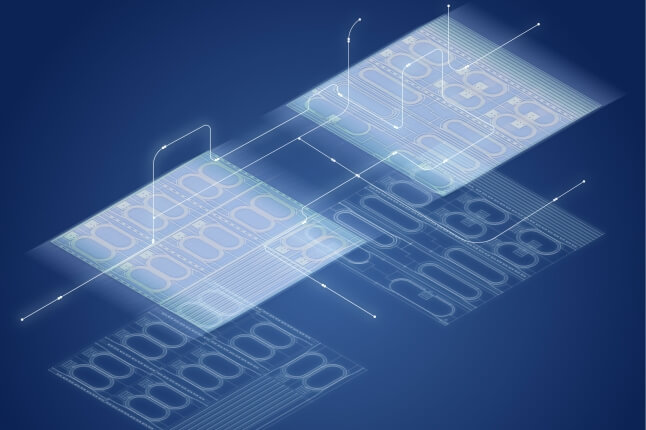
To mimic the circular hook-like attachment organ found in several species of intestinal tapeworms as an initial proof of concept, the researchers used a multi-material, layer-by-layer fabrication method inspired by the printed circuit board industry. One of the key design features of the mechanism is its radially symmetrical architecture, which allowed for the creation of a biologically accurate range of motion from simple flat components.
“Employing relatively simple linkage mechanisms allows for the use of laminate manufacturing processes, which offers several advantages over conventional fabrication approaches," said Gabriel Maquignaz, a visiting graduate student from the Swiss Federal Technology Institute of Lausanne, and the paper’s first author.
“For example, the devices can be manufactured flat and then quickly and easily folded into their final 3D geometries using a largely automated pop-up book-like process,” said Mike Karpelson, a senior staff electrical engineer at SEAS and an expert in this fabrication workflow.
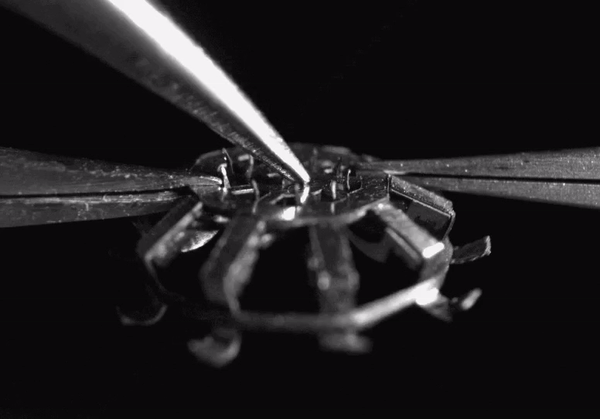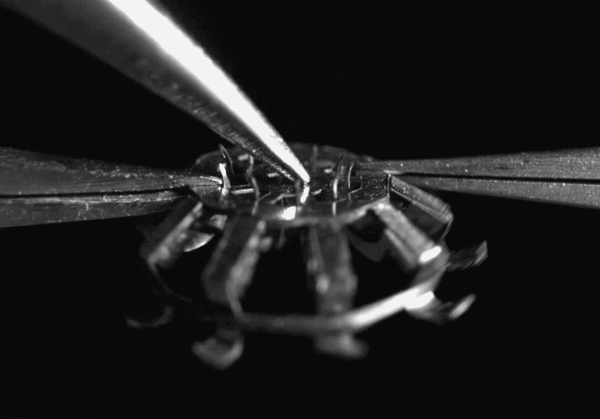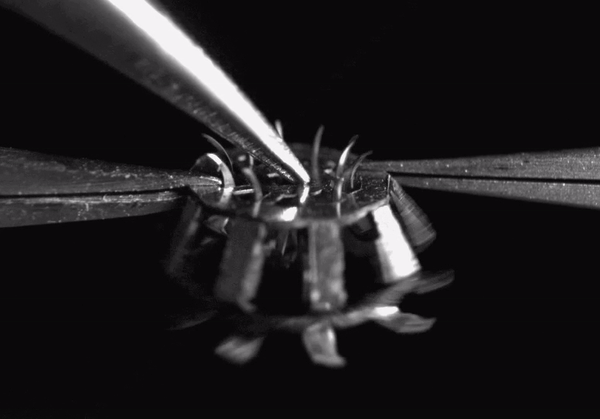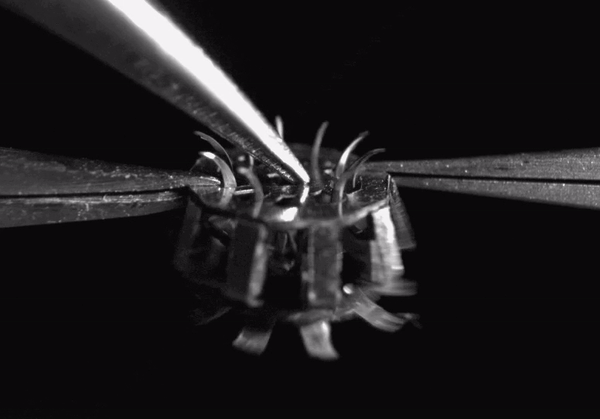
High-speed video (8000 frames per second) showing hook deployment in the tapeworm-inspired mechanism. Normally this would occur once the device contacts a tissue surface, but for clarity, here it was triggered from above with a pair of forceps. The entire deployment process takes less than 1 millisecond
Furthermore, due to its rapid turnaround time and the small size of the fabricated devices, this manufacturing approach provides a low-waste prototyping method during the device research and development phases.
The final device design contains rigid stainless steel structural components adhesively bonded to polymer hinges. The entire device measures less than 5 millimeters in diameter when deployed and weighs only 44 micrograms. When it comes in contact with a tissue surface, a trigger mechanism is activated which causes the anchoring hooks to rotate out and penetrate the adjacent soft tissue. Since each hook follows a curved trajectory, it only punctures the skin immediately along the path of penetration -just like tapeworm hooks- causing minimal tissue damage. Because of the device’s small size and its integrated elastomer spring, the hooks can be deployed in less than 1 millisecond.
The authors further add that due to the relative simplicity and adaptability of this manufacturing method, the fabricated devices could be further scaled down in size for future iterations.
“We’re really excited about applying the lessons learned from these studies to further broaden the design space to include other parasitic body plans, and other biological tissues and therapeutic applications,” said Rachel Zoll, a doctoral candidate at SEAS specializing in biomedical device design, and the article’s second author.
“One of the most intriguing aspects of this research effort is that it provides a much-needed experimental testbed for exploring how parasite holdfast anatomy influences human pathology at the point of attachment,” said Armand Kuris, a parasitology professor at UC Santa Barbara, who was not involved in the study. “This represents a largely unexplored aspect of medical parasitology, and I’m eager to see where this research leads.”
Beyond the biomedical applications that were the primary focus of the article, the authors also envision the utilization of this technology in non-medical applications ranging from reversibly adhesive tags for wildlife monitoring, to sensing platforms for textile-based materials.
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 2140743
Topics: Bioengineering, Electrical Engineering, Materials Science & Mechanical Engineering
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Robert J. Wood
Harry Lewis and Marlyn McGrath Professor of Engineering and Applied Sciences
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu