News
Howard Stone, a former SEAS faculy member and now Dixon Professor in Mechanical and Aerospace Engineering at Princeton University, explains the science of pizza to a lecture hall packed with local families during the annual holiday lecture. (Photo by Oleksandr Babii/SEAS Communications)
In the lecture halls of the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), many questions are asked every day. One question that is seldom posed is, “Who likes pizza?”
On Dec. 9, the lecture hall erupted into cheers at this question, but these cheers came from pupils who are a bit younger than the average university student. Kids of all ages and their families, 370 participants in total, attended the 2018 Holiday Science Lecture to learn about the science of pizza from Howard Stone, a former SEAS faculty member and now Dixon Professor in Mechanical and Aerospace Engineering at Princeton University.
Stone and research assistant Daniel Rosenberg began by demonstrating how liquid milk becomes crosslinked to form solid clumps of cheese. Rosenberg prepared solutions of sodium alginate, a short-chain polymer, to be poured into two different salt solutions, a sodium solution and a calcium chloride solution. Stone explained that each sodium ion has one positive charge, so it can only interact with one alginate molecule. Calcium has a positive charge of two, so it can interact with two alginate molecules to form a longer polymer. When Rosenberg poured a solution of sodium alginate into the sodium solution, nothing appeared to happen. However, when he poured a solution of sodium alginate into the calcium solution, the audience gasped in surprise as the sodium alginate formed webby strands in the solution.

Research assistant Daniel Rosenberg displays the webby strands that formed when he poured a solution of sodium alginate into a sodium solution. (Photo by Oleksandr Babii/SEAS Communications)
To show the crosslinking that happens in cheese, Stone and Rosenberg put a drop of milk under the microscope. Projected onto the large screen, droplets of fat swirled around in water. Once Rosenberg added a drop of vinegar, the room filled with exclamations as kids watched the fat droplets clump together on the big screen. Stone and Rosenberg then prepared a cheesecloth to make cheese at a much larger scale for kids to try at the end of the lecture.
Kids wearing t-shirts labeling them as monomers and crosslinkers were called from the audience to illustrate this process. The monomers roamed freely “in solution” until the crosslinkers were added to the solution to hold the hands of two different kids, forming a polymer that snaked across the front of the lecture hall.
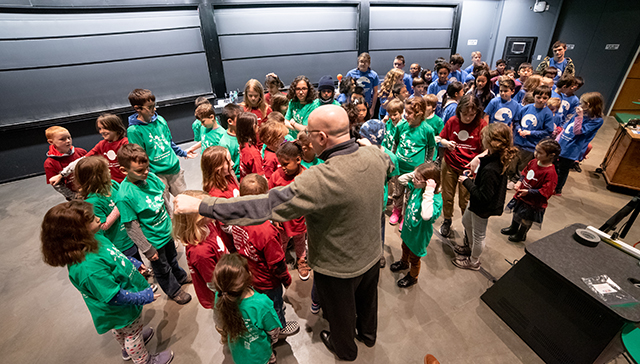
Stone gives directions as the children act out a chemcial reaction. (Photo by Oleksandr Babii/SEAS Communications)
Stone then explained how to make the crust. Children learned about the importance of having experimental controls, as they each conducted their own experiment at their seats. Each child was given a sugar solution with yeast and one without yeast. Each vial of solution was attached to a deflated balloon. As the yeast consumed the sugar, releasing gases, one of the balloons slowly expanded, signalling the presence of the yeast.
In the final live demonstration, kids were called upon to illustrate how yeast produces holes in bread. Children joined hands at the front of the lecture hall to represent one long polymer. Then, blue-shirted “enzyme" kids were released to tap apart the hands of the “polymer” children with a “chomp chomp.” For the second part of the demonstration, they were instructed to crowd around the instructors, and the adults then pushed the kids gently back to form bubbles in the “bread,” like yeast would.

This experiment showed children the role yeast plays in pizza dough. (Photo by Oleksandr Babii/SEAS Communications)
Two “enzymes,” Mina Moller and Zoe Isabelle, 11-year-olds from Newton, have been coming to the holiday science lecture for the past three years. Moller said she liked the demonstrations, and her brother, 11-year-old Otto, said they would definitely try to make cheese at home.
“It’s cool you can make cheese from two ingredients,” said Isabelle.
At the end of the lecture, the cheese was finally ready, and kids crowded around for a taste of science.

The lecture over, these young scientists dug into cheese Stone and Rosenberg made during the program. (Photo by Oleksandr Babii/SEAS Communications)
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Adam Zewe | 617-496-5878 | azewe@seas.harvard.edu
