News
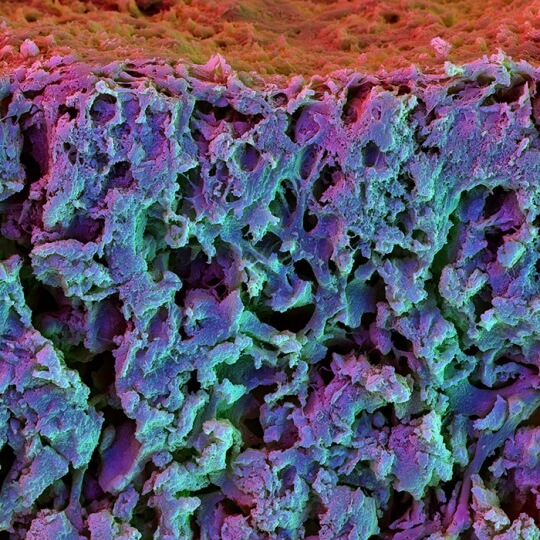
Scanning electron microscopy image of the cross section of a fast relaxing hydrogel containing mesenchymal stem cells. The cells differentiated into osteoblasts and integrated in the matrix.
Researchers at the Harvard John A. Paulson School of Engineering and Applied Sciences and The Wyss Institute for Biologically Inspired Engineering have developed a new, more precise way to control the differentiation of stem cells into bone cells. This new technique has promising applications in the realm of bone regeneration, growth and healing. The research led by David Mooney, the Robert P. Pinkas Family Professor of Bioengineering at SEAS, was published in Nature Materials.
A cell’s microenvironment, the network of proteins and polymers that surrounds and connects cells within tissues, impacts a range of cellular behaviors, including stem cell differentiation. For about a decade, researchers have been able to direct the fate of stem cells by tuning the stiffness of its microenvironment, also known as the extracellular matrix. The problem with only tuning stiffness is that it assumes the environment behaves like an elastic material, like rubber. When a strain or deformation is exerted on an elastic material, elastic energy is stored and when the deformation is released, the material bounces back to its original shape like a rubber band.
Of course, in nature, extracellular matrices are not elastic, they are viscoelastic. Viscoelastic materials, like chewing gum, relax stress and dissipate energy over time when a deformation is applied.
Mooney and his team decided to mimic the viscoelasticity of living tissue by developing hydrogels with different stress relaxation responses. When they put stem cells into this viscoelastic microenvironment and tuned the rate at which the gel relaxed, they observed dramatic changes in the behavior and differentiation of the cells.
“We found that with increasing stress relaxation, especially combined with increased stiffness in the hydrogel, there is an increase of osteogenic — bone cell — differentiation,” said Luo Gu, postdoctoral fellow in the Mooney lab and co-first author. “With increased stress relaxation, there was also a decrease in the differentiation into fat cells. This is the first time we’ve observed how matrix stress relaxation impacts stem cell differentiation in 3D.”
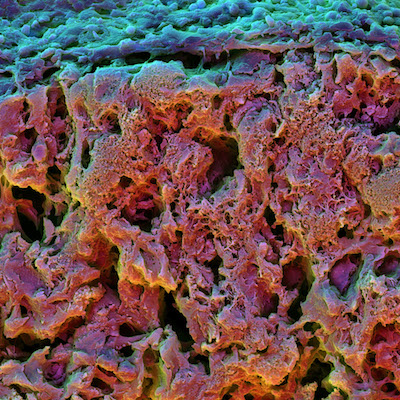 Not only did increased stress relaxation dramatically increase early osteogenic differentiation but those cells continued to grow as bone cells weeks after their initial differentiation and formed an interconnected mineralized matrix rich in collagen, key structural features of bone.
Not only did increased stress relaxation dramatically increase early osteogenic differentiation but those cells continued to grow as bone cells weeks after their initial differentiation and formed an interconnected mineralized matrix rich in collagen, key structural features of bone.
“This work both provides new insight into the biology of regeneration, and is allowing us to design materials that actively promote tissue regeneration,” said Mooney, who is also a core faculty member of the Wyss Institute.
Harvard’s Office of Technology Development has filed a patent application and is actively exploring commercial opportunities for the technology.
One reason that fast-relaxing microenvironments promote more osteogenesis and form bone is that cells inside these matrices can mechanically remodel the matrix and more easily change shape, said Ovijit Chaudhuri, former postdoctoral fellow in the Mooney lab and co-first author.
“Imagine being trapped in a block of rubber,” Chaudhuri said. “Every movement is opposed by the elasticity of the rubber. But if instead of rubber you are trapped in Silly Putty, which relaxes stress very quickly and is malleable, you can remodel the putty and move around. In our experiments, we found that faster stress relaxation allowed strikingly different cell morphologies.”
It may seem counter intuitive that bone cells need fast-relaxing environments to grow into bone, which is very stiff and elastic. However, the team observed that the microenvironment around bone fractures is very similar to the fastest-relaxing hydrogel the team developed in the lab.
“Coagulated marrow and fracture hematoma are viscoelastic and have fast stress relaxation behavior,” Gu said. “This may be an indication that in the natural environment, when a bone fracture is healing, it needs a really fast stress relaxation matrix to assist in bone formation.”
The next stage of the research is to test fast-relaxing hydrogels in vivo, to see if they promote bone healing.
“In addition to introducing a new concept to the fields of mechanobiology and regenerative medicine, I expect this work will lead to an explosion of new ideas and research to examine how a number of other material mechanical properties influence cell behavior,” said Mooney.
The paper was co-authored by Darinka Klumpers, Max Darnell, Sidi A. Bencherif, James C. Weaver, Nathaniel Huebsch, Hong-pyo Lee, Evi Lippens and Georg N. Duda. This research was funded by the National Institute of Health, the Einstein Foundation Berlin and Harvard Materials Research Science and Engineering Center.
Topics: Health / Medicine
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu