News
Harvard bioengineers have developed a human airway muscle-on-a-chip that could be used to test new drugs because it accurately mimics the way smooth muscle contracts in the human airway.(Image reproduced by permission of Kevin Kit Parker from Lab Chip [2014] 14, 3925. Image by Karaghen Hudson, Disease Biophysics Group, Harvard SEAS & Wyss Institute.)
Cambridge/Boston, Mass – September 23, 2014 – The majority of drugs used to treat asthma today are the same ones that were used 50 years ago. New drugs are urgently needed to treat this chronic respiratory disease, which causes nearly 25 million people in the United States alone to wheeze, cough, and find it difficult at best to take a deep breath.
But finding new treatments is tough: asthma is a patient-specific disease, so what works for one person doesn’t necessarily work for another, and the animal models traditionally used to test new drug candidates often fail to mimic human responses, costing tremendous money and time.
Hope for healthier airways may be on the horizon thanks to a Harvard University team that has developed a human airway muscle-on-a-chip that could be used to test new drugs because it accurately mimics the way smooth muscle contracts in the human airway, under normal circumstances and when exposed to asthma triggers. As reported in the journal Lab on a Chip, it also offers a window into the cellular and even subcellular responses within the tissue during an asthmatic event.
“Every year asthma costs many tens of billions of dollars, significant productivity due to lost work and school days, and even lives,” said senior author Kevin Kit Parker, Tarr Family Professor of Bioengineering and Applied Physics at the Harvard School of Engineering and Applied Sciences (SEAS) and a Core Faculty member at Harvard’s Wyss Institute for Biologically Inspired Engineering. Parker leads development of the Wyss Institute’s heart-on-a-chip technology as well and is specifically interested in diseases that affect children. “We were thrilled with how well the chip recapitulated the functioning of the human airway,” he said.
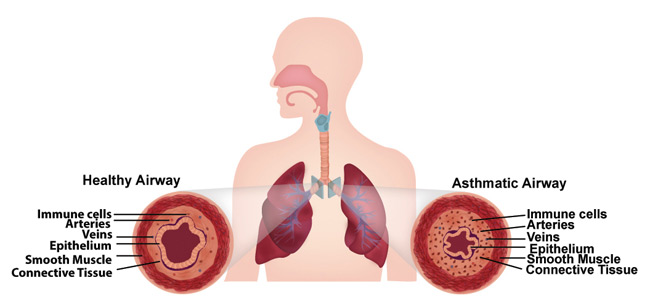
Asthmatic airways are constricted. (Image credit Wyss Institute and Harvard SEAS.)
The chip, a soft polymer well that is mounted on a glass substrate, contains a planar array of microscale, engineered human airway muscles, designed to mimic the laminar structure of the muscular layers of the human airway.
To mimic a typical allergic asthma response, the team first introduced interleukin-13 (IL-13) to the chip. IL-13 is a natural protein often found in the airway of asthmatic patients that mediates the response of smooth muscle to an allergen.
Then they introduced acetylcholine, a neurotransmitter that causes smooth muscle to contract. Sure enough, the airway muscle on the chip hypercontracted—and the soft chip curled up—in response to higher doses of the neurotransmitter.
They achieved the reverse effect as well and triggered the muscle to relax using drugs called β-agonists, which are used in inhalers.
Significantly, they were able to measure the contractile stress of the muscle tissue as it responded to varying doses of the drugs, said lead author Alexander Peyton Nesmith, a Ph.D./M.D. student at Harvard SEAS and the University of Alabama at Birmingham. “Our chip offers a simple, reliable and direct way to measure human responses to an asthma trigger,” he said.
The team then investigated what happened on a cellular level in response to the IL-13 and confirmed, for example, that the smooth muscle cells grew larger in the presence of IL-13 over time—a structural hallmark of the airways in asthma patients as well. They also documented an increased alignment of actin fibers within smooth muscle cells, which is consistent with the muscle in the airway of asthma patients. Actin fibers are super-thin cellular components involved in muscle contraction.
Next they observed how IL-13 changes the expression of contractile proteins called RhoA proteins, which have been implicated in the asthmatic response, although the details of their activation and signaling have remained elusive. To do this they introduced a drug called HA1077, which is not currently used to treat asthmatic patients—but targets the RhoA pathway. It turns out that the drug made the asthmatic tissue on the chip less sensitive to the asthma trigger—and preliminary tests indicated that using a combined therapy of HA1077 plus a currently approved asthma drug worked better than the single drug alone.
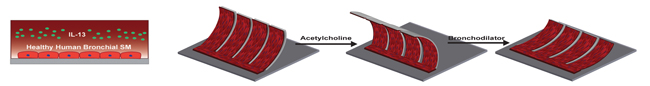
The researchers mimicked an asthmatic airway on their airway muscle-on-a-chip by first introducing Interleukin-13 (IL-13), a natural protein often found in the airway of asthmatic patients. Then they used a neurotransmitter called acetylcholine to trigger the smooth muscle to hypercontract, and a bronchodilator used in inhalers called a β-agonist to elicit muscle relaxation. (Image credit Wyss Institute and Harvard SEAS.)
“Asthma is one of the top reasons for trips to the emergency room, particularly for children, and a large segment of the asthmatic population doesn’t respond to currently available treatments,” said Donald Ingber, who is the founding director of the Wyss Institute, Judah Folkman Professor of Vascular Biology at Harvard Medical School and Children’s Hospital Boston and Professor of Bioengineering at Harvard SEAS. “The airway muscle-on-a-chip provides an important and exciting new tool for discovering new therapeutic agents.”
This work was funded by the National Institutes of Health and Harvard SEAS.
Topics: Health / Medicine, Bioengineering
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Kit Parker
Tarr Family Professor of Bioengineering and Applied Physics
Press Contact
Caroline Perry