News
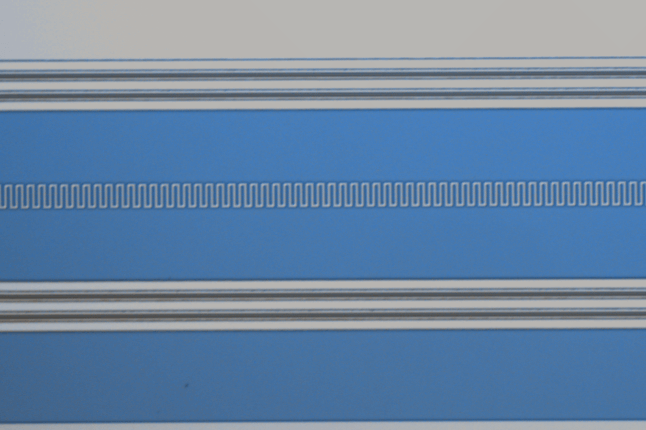
Microscope images of crystals grown on water droplets. These structures might provide insights into the formation of curved nanoscale objects such as viruses. (Image courtesy of Guangnan Meng and Vinothan N. Manoharan.)
Scientists have studied crystallization since the time of Galileo, so it’s easy to imagine there’s nothing new to learn about the process.
Harvard researchers might beg to differ.
A new study has uncovered a previously unseen phenomenon — that curved surfaces can dramatically alter the shape of crystals as they form. The finding could have applications ranging from applying coatings to nanoparticles used in industry to aiding in drug delivery, and may even help shed light on how viruses assemble. The work, conducted by researchers at Harvard’s Materials Research Science and Engineering Center and funded by the National Science Foundation, is described in a Feb. 7 paper in Science.
To investigate how curved surfaces affect crystallization, Vinothan N. Manoharan, Gordon McKay Professor of Chemical Engineering at the Harvard School of Engineering and Applied Sciences (SEAS) and professor of physics, worked with physics postdoc Guangnan Meng to develop a system in which nanoscale colloidal particles were injected into water droplets. As the particles — about 10,000 times larger than atoms or molecules — organized themselves into crystalline structures, researchers were able to observe the process in real time.
“If you have the particles on a flat surface, like a piece of glass, they form a regular lattice, and they’re compact, with no preferred direction for crystal growth,” Manoharan said. “On a curved surface, however, they form a very different pattern. It looks like strips — almost like ribbons — and they branch out from different points.”
Importantly, Manoharan said, researchers found that changing the curvature of the surface — by changing the radius of the water droplet — resulted in changes to the crystalized “ribbons.”
“As the droplet gets less curved, the width of those ribbons gets bigger,” he explained. “That was one of the hints that what we were seeing was the result of the curvature.”
Manoharan stressed that for the new process to work, the crystals need to be able to “feel” the curvature of the structure they grow on.
“This may not occur when we look at crystals made from atoms or molecules because the atoms and molecules are so small compared to the curved surface — they will not feel the curve, just like we don’t feel the curvature of the Earth when we walk around,” he said. “But if you had an atomic or molecular crystal growing on a nanoscale object, then it’s likely it would feel the curve, and when we looked at the literature, we were surprised to find reports of patterns in nanoscale crystals that look a great deal like the structures we saw.”
Though the crystals’ appearance initially seemed similar to that found in snowflakes, Manoharan and Meng believe crystals on curved surfaces form through an entirely different process. The distinctive structure of snowflakes occurs, in part, due to the fact that the crystals form extremely fast, meaning water molecules don’t have time to form more compact structures. By comparison, the crystals created in water droplets by the Harvard team in some cases took hours to form.
Meng and Manoharan brought their results to David R. Nelson, Arthur K. Solomon Professor of Biophysics and professor of physics and applied physics, and Jayson Paulose, a graduate student in applied physics at SEAS. The two were able to create a model that helped explain the structure of the crystals.
Read the entire article in the Harvard Gazette
Topics: Applied Physics
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Scientist Profiles
Vinothan N. Manoharan
Wagner Family Professor of Chemical Engineering and Professor of Physics
David R. Nelson
Arthur K. Solomon Professor of Biophysics and Professor of Physics and Applied Physics
Press Contact
Caroline Perry