News
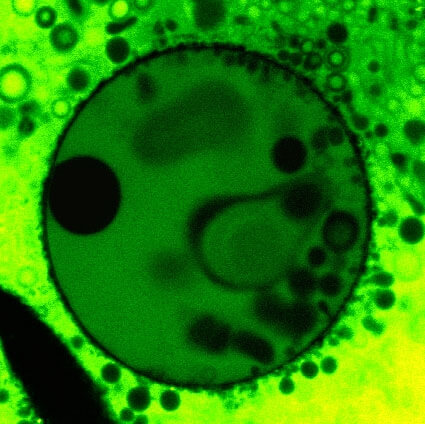
This false-color confocal image shows fatty-acid liposomes compartmentalized in a clay vesicle.The clay vesicles are approximately 100 microns in diameter, and the pores in the clay tend to range in size from about 50 to 100 nanometers (nm), with a very small percentage of vesicles able to exclude molecules as small as 17 nm. Fatty acid molecules, thought to be the components of primitive organic cell membranes, have been shown to travel through the pores into the vesicle and automatically assemble into micron-sized liposomes.Photo courtesy of Anand Bala Subramaniam.
Cambridge, Mass. – February 7, 2011 – A team of applied physicists at Harvard’s School of Engineering and Applied Sciences (SEAS), Princeton, and Brandeis have demonstrated the formation of semipermeable vesicles from inorganic clay.
The research, published online this week in the journal Soft Matter, shows that clay vesicles provide an ideal container for the compartmentalization of complex organic molecules.
The authors say the discovery opens the possibility that primitive cells might have formed inside inorganic clay microcompartments.
"A lot of work, dating back several decades, explores the role of air bubbles in concentrating molecules and nanoparticles to allow interesting chemistry to occur,” says lead author Anand Bala Subramaniam, a doctoral candidate at SEAS.
"We have now provided a complete physical mechanism for the transition from a two-phase clay–air bubble system, which precludes any aqueous-phase chemistry, to a single aqueous-phase clay vesicle system," Subramaniam says, "creating a semipermeable vesicle from materials that are readily available in the environment."
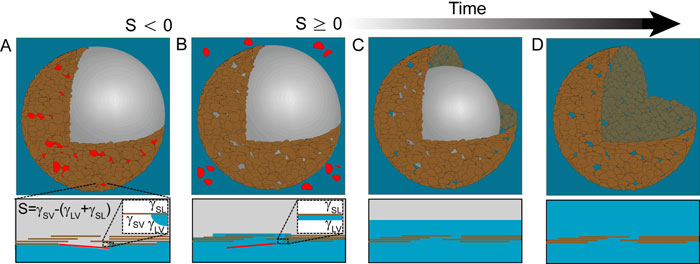
The authors' schematic of clay vesicle formation, showing a cut-away view of the clay shell and dissolving bubble at the top, and a view of the water-air interface at the bottom. Image courtesy of Anand Bala Subramaniam.
"Clay-armored bubbles" form naturally when platelike particles of montmorillonite collect on the outer surface of air bubbles under water.
When the clay bubbles come into contact with simple organic liquids like ethanol and methanol, which have a lower surface tension than water, the liquid wets the overlapping plates. As the inner surface of the clay shell becomes wet, the disturbed air bubble inside dissolves.
The resulting clay vesicle is a strong, spherical shell that creates a physical boundary between the water inside and the water outside. The translucent, cell-like vesicles are robust enough to protect their contents in a dynamic, aquatic environment such as the ocean.
Microscopic pores in the vesicle walls create a semipermeable membrane that allows chemical building blocks to enter the “cell,” while preventing larger structures from leaving.
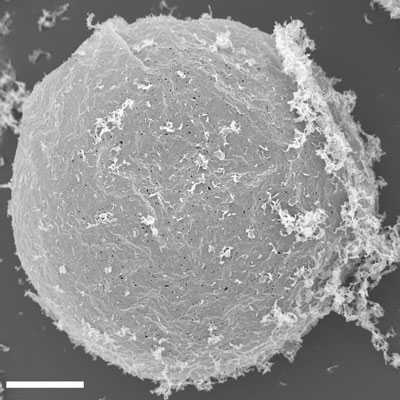
This SEM image shows the exterior surface of a clay vesicle. Photo courtesy of Anand Bala Subramaniam.
Scientists have studied montmorillonite, an abundant clay, for hundreds of years, and the mineral is known to serve as a chemical catalyst, encouraging lipids to form membranes and single nucleotides to join into strands of RNA.
Because liposomes and RNA would have been essential precursors to primordial life, Subramaniam and his coauthors suggest that the pores in the clay vesicles could do double duty as both selective entry points and catalytic sites.
“The conclusion here is that small fatty acid molecules go in and self-assemble into larger structures, and then they can’t come out,” says principal investigator Howard A. Stone, the Dixon Professor in Mechanical and Aerospace Engineering at Princeton, and a former Harvard faculty member. “If there is a benefit to being protected in a clay vesicle, this is a natural way to favor and select for molecules that can self-organize.”
Future research will explore the physical interactions between the plate like clay particles, and between the liquids and the clay. The researchers are also interested to see whether these clay vesicles can, indeed, be found in the natural environment today.
“Whether clay vesicles played a significant role in the origins of life is of course unknown," says Subramaniam, "but the fact that they are so robust, along with the well-known catalytic properties of clay, suggests that they may have had some part to play.”
Subramaniam and Stone’s coauthors include Jiandi Wan, of Princeton University, and Arvind Gopinath, of Brandeis University.
The research was funded by the Harvard Materials Research Science and Engineering Center and supported by the Harvard Center for Brain Science Imaging Facility.
###
Video and high-resolution photos are available upon request.
Topics: Applied Physics
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Caroline Perry